cuscuta reviews
C. australis seedlings have no roots. To survive the time window of a few days that are needed for finding suitable hosts, they must be able to minimize water loss to counteract drought stress.
The Parasitic Plant Cuscuta australis Is Highly Insensitive to Abscisic Acid-Induced Suppression of Hypocotyl Elongation and Seed Germination
Affiliations College of Plant Science and Technology, Huazhong Agricultural University, Wuhan, Hubei, China, Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, China
Affiliation Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, China
Affiliation Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, China
Affiliation Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, China
Affiliation College of Plant Science and Technology, Huazhong Agricultural University, Wuhan, Hubei, China
Affiliations Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, China, Yunnan Key Laboratory for Wild Plant Resources, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, China
Planting non-host grass crops (e.g., corn, sorghum), winter crops (e.g., winter wheat, broccoli, legumes), and transplanted trees with bark (e.g., pecan) can be effective in managing dodder in an infested area. However, certain broadleaf weeds, such as pigweed, puncturevine, lambsquarter, Russian thistle, and field bindweed, serve as dodder host plants and will need to be controlled as part of a successful dodder management strategy. Furthermore, due to the longevity of dodder seed, once a host crop is planted again, fields need to be monitored regularly, and new dodder plants must be removed immediately.
Description and Life Cycle
In the spring, dodder seeds germinate near the soil surface and send up slender, thread-like twining stems varying in color from pale green to yellow or orange and without any cotyledons (seed leaves). The slender, leafless, thread-like stem sways or rotates slowly until it touches the stem or leaf of another plant and begins to wind around it (Figures 1 and 2). On a host plant, the dodder stem will immediately form small appendages called haustoria (tiny sucker-like roots), which penetrate the stems or leaves so that dodder can extract its necessary growth requirements. Soon after attaching to a host plant, the lower end of the dodder withers and breaks its connection with the ground, while the upper part of the stem grows rapidly, often forming dense, stringy masses. However, if the dodder seedlings are unable to make physical contact with a susceptible host plant soon after germination, they will not survive.

Figure 1. Dodder parasitizing a puncturevine (Tribulus terrestris) plant.

Figure 2. Dodder stems wind around the plants they parasitize. (Photo by J.M. DiTomaso; used with permission.)
Dodder flowers are numerous, tiny, and whitish to pinkish, and form in small clusters along the stems, generally from May to October depending on the species and location. Each flower forms a small, globular seedpod with 2 to 4 seeds (Figures 3 and 4). The seeds have rough coats and vary in size depending on the species, and may be able to survive over 20 years in the soil.

Figure 3. Dodder flowers in small clusters along the stems.

Figure 4. Close-up of a dodder flower cluster. (Photo by J.M. DiTomaso; used with permission.)
This study randomly established 10,000 validation sites, and used the actual point of existence to run the model. To obtain the best model, the Maxent model was set as follows: (1) the regularization multiplier (beta) selected based on corrected Akaike information criterion (AICc) was set to 1, 2, 5, 10, 15, and 20 [19, 72, 73]. (2) A 10 cross-validation approach was used as replicated run type [22]. (3) A complementary log–log (cloglog) transformation was used to produce an estimate of the habitat suitability of weeds [67]. (4) The resolutions of environmental variables were set to 2.5, 5.0, and 10.0 arc min. The Maxent model was run 10 times repeatedly. Each run randomly selected 90% of the distribution information points as training set, while the remaining 10% of the samples were used for the test. The threshold rule selected equal training sensitivity and specificity. The output format of Maxent was selected automatically depending on the particular sample size of the occurrence records according to a method developed by Phillips and Dudik [62]. The auto feature setting was selected and the regularization multiplier (beta) was 1. In addition, the environmental variable was set to 2.5 min.
Results
Model performance and contribution of variables
Ecological modeling yielded an average AUC value of 0.951, while the TSS index was 0.887, classifying the model as very satisfactory. The six bioclimatic variables of annual mean temperature (Bio1), isothermality (Bio3), temperature seasonality (Bio4), precipitation seasonality (Bio15), precipitation of warmest quarter (Bio18), and precipitation of coldest quarter (Bio19) were selected to establish the model (Table 1). Additional file 1: Fig. S1 shows the results of the Jackknife test of the variable contribution by Maxent. When used independently, Bio1, Bio3, Bio15, and Bio18 provided very high gains (> 0.40), indicating that these four variables contained more useful information than the other variables. Bio4 and Bio19 achieved very low yields when used alone, and did not contain much information. Therefore, Bio1, Bio3, Bio15, and Bio18 were identified as important climatic factors that influence the suitable habitat of C. chinensis.
Response of variables to suitability
The response curves of C. chinensis to the six assessed bioclimatic variables are shown in Additional file 2: Fig. S2. As shown in Additional file 2: Fig. S2a, when Bio1 is below 5 °C, the probability that C. chinensis exists is extremely low (below 0.5, indicating low probability). With increasing temperature, the probability for C. chinensis to exist gradually increased, and reached the maximum at 22 °C with a probability of existence as high as 0.7. When Bio1 ranges from 4 to 37 °C, the survival rate of C. chinensis was high (
0.5). Therefore, the optimum annual mean temperature of C. chinensis ranges from 4 °C to 37 °C.
As shown in Additional file 2: Fig. S2b, when Bio3 ranged from 0 to 45, the survival probability of C. chinensis exceeded 0.5, indicating a benefit for the survival of C. chinensis. Therefore, the optimum isothermality of C. chinensis should remain below 45.
As shown in Additional file 2: Fig. S2c, when the temperature seasonality of Bio4 was
500 or less, the existence probability of C. chinensis was extremely low. Furthermore, from 4000 to 25,000, the survival probability of C. chinensis first increased and then decreased, and the survival probability of C. chinensis decreased to above 0.5. Therefore, the optimal temperature seasonality of C. chinensis is 4000–25,000.
As shown in Additional file 2: Fig. S2d, when Bio15 exceeds 25, the survival probability of C. chinensis rapidly increased and reached a peaked at around 80 (
0.72). From 50 to 130, the survival probability of C. chinensis exceeded 0.5. Therefore, the optimal precipitation seasonality ranges from 50 to 130.
As shown in Additional file 2: Fig. S2e, with increasing Bio18, the survival probability of C. chinensis gradually increased and peaked at around 500 mm. Beyond 500 mm, the survival probability of C. chinensis deceased and reached a minimum at around 1400 mm. Furthermore, the optimal precipitation of the warmest quarter ranges from 300 to 1000 mm, and from 2500 to 3500 mm with the survival probability of C. chinensis exceeding 0.5.
As shown in Additional file 2: Fig. S2f, when Bio19 ranged from 0 to 2000 mm, the existence probability of C. chinensis exceeded 0.5. Therefore, the optimal precipitation of C. chinensis in the coldest season ranges from 0 mm to 2000 mm and the survival probability exceeds 0.5.
Model application
Global C. chinensis distribution
The global C. chinensis distribution is shown in Fig. 1a. In Asia, C. chinensis are mainly distributed at latitudes ranging from 20° N to 50° N, which includes central, eastern, and southern China (Fig. 1c). C. chinensis also has a small distribution in Japan, India, Afghanistan, Pakistan, Myanmar, Vietnam, Bangladesh, and Turkey as well as minor occurrences in Australia (Fig. 1a, c). However, no distribution was found on Europe, Africa, and America (Fig. 1a).
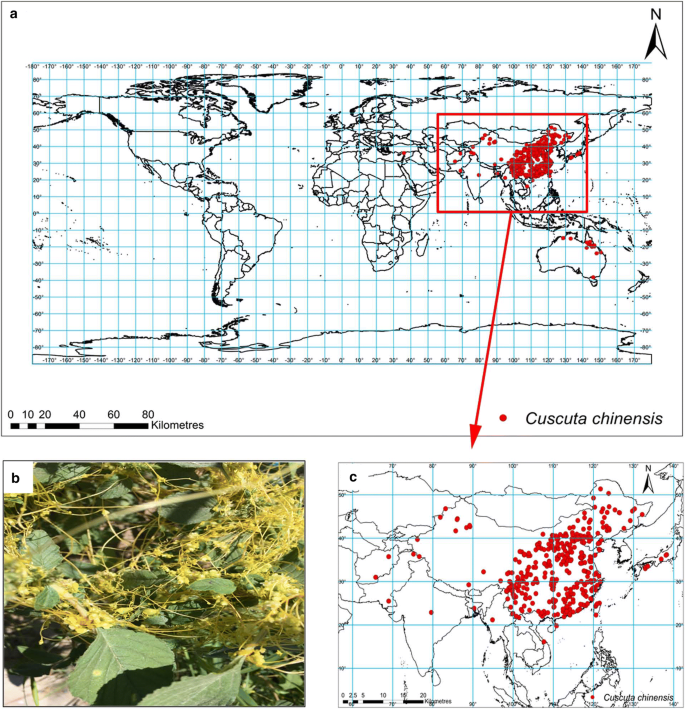
Global distribution (a), photo (b) and concentrated distribution (c) of Cuscuta chinensis. Geographical base map data were downloaded from the national basic geographic information system (http://www.diva-gis.org/)
Habitat suitability simulation for three historical periods
The simulation results of the C. chinensis habitat suitability during three historical periods (last glacial maximum, mid-Holocene, and 1960–1990) are shown in Fig. 2. From the perspective of space, suitable areas for C. chinensis during these three periods concentrated in the central, northern, southern, and eastern parts of China. These areas have a survival probability above 0.5, indicating that C. chinensis in these region benefitted from moderate or relatively high suitability. Compared with the last glacial maximum, the paleoclimatic prediction of the Holocene mid-term CCSM4 climate model indicates that the position in the mid-Holocene changed; moreover, it indicated that the size of the predicted distribution increased. From the mid-Holocene to 1960–1990, the global habitat suitability of C. chinensis gradually decreased, and the area with medium and relatively higher fitness (> 0.5) gradually decreased. From the last glacial maximum to the mid-Holocene, the total area with suitability above 0.75 increased by 0.5689 million km 2 (i.e., by 25.09%). The area with higher fitness (> 0.75) during the mid-Holocene reached 2.8362 million km 2 , accounting for 1.9% of the global total area. However, from the mid-Holocene to 1960–1990, the total area with suitability above 0.75 decreased by 0.0797 million km 2 (i.e., by 2.81%). During the period of 1960-1990, the area with high fitness (> 0.75) was 2.7565 million km 2 , accounting for 1.85% of the global total area. From the last glacial maximum to the mid-Holocene, the total area with suitability of 0.5-1 increased by 0.0875 million km 2 , while from the mid-Holocene to 1960–1990, the total area with suitability of 0.5–1 decreased by 0.0759 million km 2 (Table 2).
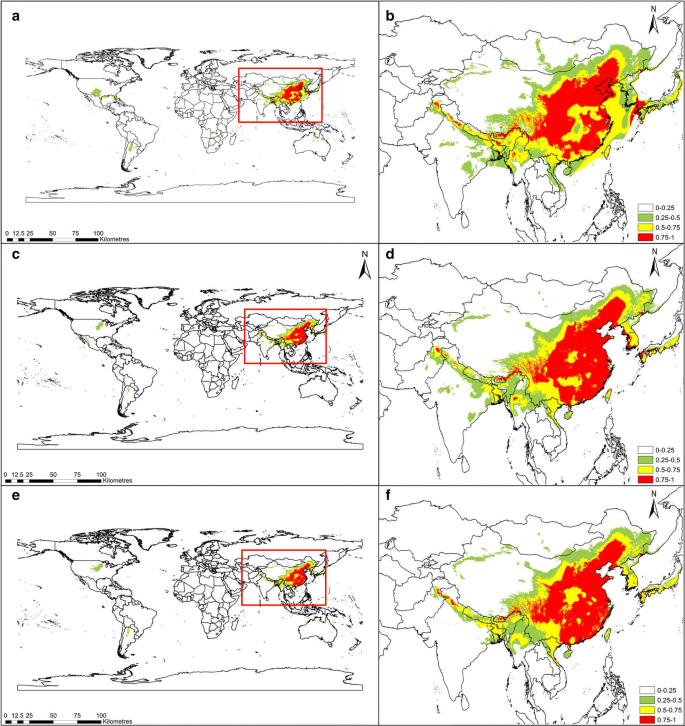
Habitat suitability distribution of Cuscuta chinensis for three historical periods. Last glacial maximum (a, b), mid-Holocene (c, d), and 1960–1990 (e, f). Geographical base map data were downloaded from the national basic geographic information system (http://www.diva-gis.org/)
Suitable habitat distributions under global warming scenarios
The computed results for the C. chinensis habitat suitability in RCP2.6 and RCP8.5 are shown in Figs. 3 and 4, respectively. The suitable habitats of C. chinensis decreased in response to climatic warming. In RCP8.5, the total area with intermediate suitability and high suitability for the survival of C. chinensis was less than that of RCP2.6. In RCP2.6, the C. chinensis suitabilities of northern, central, and southern China, North Korea, and the coastal areas of Japan all exceed 0.75. However, the suitabilities of southern Africa, the central and southern parts of North America, and South America ranged between 0.25 and 0.5, while the habitat suitability of the remaining areas was below 0.25. In RCP2.6, the area with suitable habitat was below 0.25 (about 141 million km 2 ), accounting for 94.6% of the global area. Areas where the habitat suitability ranged between 0.25 and 0.5, as well as between 0.5 and 0.75 accounted for 2.0% and 1.5% of the world, respectively, with areas of about 2.9526 million km 2 and 2.2519 million km 2 , respectively. Habitats with suitability exceeding 0.75 accounted for 1.91% of the total area of the world. In RCP8.5, the area suitable for C. chinensis growth between 0.25 and 0.5 was the same as in RCP2.6, and its distribution concentrated in the central and southern parts of North America and South America. Moreover, habitats with suitability above 0.75 were also distributed in Northern China, North Korea, and the coastal areas of Japan. Compared with RCP2.6, for RCP8.5, the areas with high suitability for survival increased by 0.052 million km 2 ; however, areas with intermediate suitability and high suitability for survival decreased by 0.18 million km 2 . Areas with habitat suitability below 0.25% accounted for 94.8% of the world’s total area, with an area of 141 million km 2 . Compared with RCP2.6, this indicates an increase of 0.3298 million km 2 . Therefore, in general, habitats suitable for C. chinensis decreased in response to climate warming (Table 3).
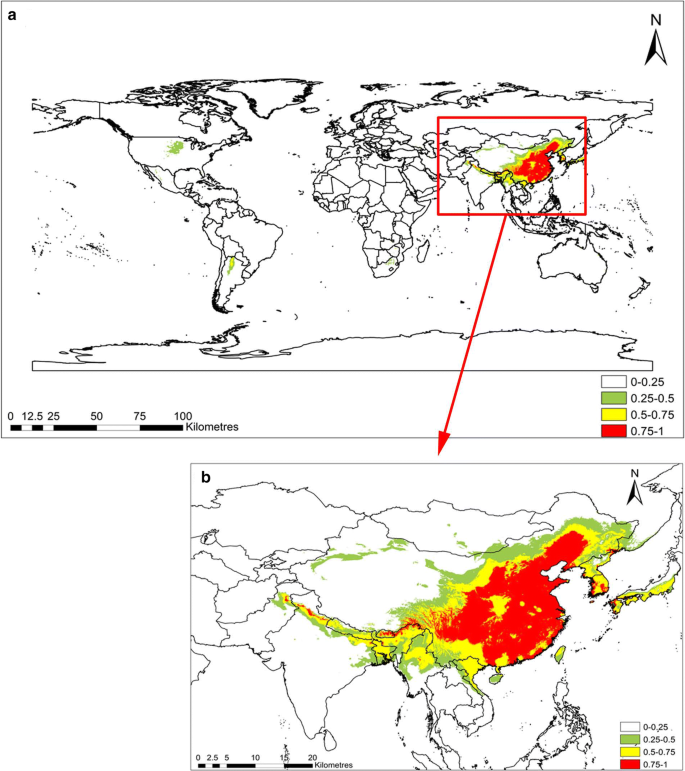
Suitable habitat distribution of Cuscuta chinensis in RCP2.6. Geographical base map data were downloaded from the national basic geographic information system (http://www.diva-gis.org/)
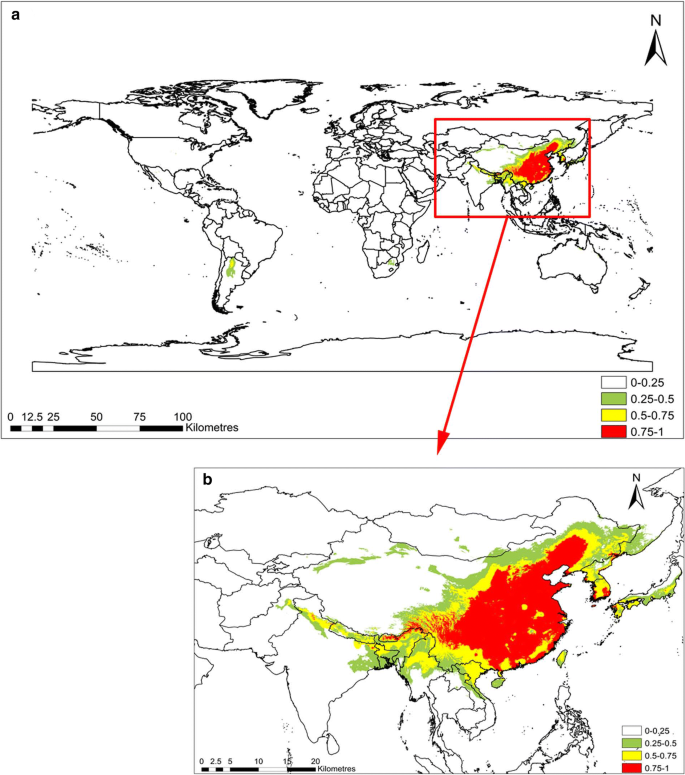
Suitable habitat distribution of Cuscuta chinensis in RCP8.5. Geographical base map data were downloaded from the national basic geographic information system (http://www.diva-gis.org/)
Although dodder is capable of limited photosynthesis, it obtains nearly all of its energy from the host plant. A dodder seedling can survive several days without a host, but if it doesn’t come into contact with one within 5 to 10 days, the seedling will die. Dodder stems that have attached to a host plant have been known to survive for several days after being detached from the host plant.
How to Manage Pests

After dodder attaches to a host, its connection to the soil withers.

Mature dodder plant with seeds.

Japanese dodder can cover entire trees with its bright yellow-gold and green stems.
Dodder, Cuscuta species, is a parasitic annual plant that infests many crops, ornamentals, native plants, and weeds. More than 150 species occur worldwide, although dodder is most prevalent in the Americas. The genus Cuscuta is in the Cuscutaceae family, but sometimes it is included in the family Convolvulaceae (morning glories).
Dodder species vary in the number of different host species they can infect. Some, such as C. salina, are in rather restricted sites such as salty marshes, flats, and ponds on just a few host plant species.
Others, such as C. pentagona (C. campestris in some publications), are found on many crop and weed species including alfalfa, asparagus, melons, safflower, sugarbeet, tomato, field bindweed (Convolvulus arvensis), lambsquarters (Chenopodium album), and pigweed (Amaranthus species). C. indecora, also has a wide host range that includes alfalfa and weeds such as field bindweed, five-hook bassia (Bassia hyssopifolia), lambsquarters, and Russian thistle (Salsola tragus).
Japanese dodder, C. japonica, which is native to Asia, recently has been found in California attacking and covering ornamental shrubs and fruit trees, with a preference toward citrus. However, Japanese dodder also can parasitize annuals, perennials, and native trees such as oaks and willows.
Table 1lists some ornamental and vegetable plants susceptible to native species of dodder.
IDENTIFICATION
Dodder has slender, twining or threadlike stems that vary from pale green to yellow or bright orange; the bright stems can be readily seen against the foliage of the host plants.
Native dodder can be leafless or have small, scalelike, triangular leaves about 1/16 inch long. The bell-shaped flowers are cream colored and about 1/8 inch long; they usually occur in clusters but occasionally are borne singly. Each flower produces a seed capsule with 2 to 3 seeds. Seeds have rough coats and vary in size depending on species but generally are about 1/16 inch in diameter. Seedlings are yellowish, threadlike, rootless, leafless stems.
Japanese dodder stems are thicker than other dodder species and resemble spaghetti. Like native dodder, Japanese dodder stems are round and twining; however, they are leafless. This species rarely flowers, but if it does, the flowers are small and pale yellow to cream. It is most likely to attack trees and shrubs.
LIFE CYCLE
Although dodder is capable of limited photosynthesis, it obtains nearly all of its energy from the host plant. A dodder seedling can survive several days without a host, but if it doesn’t come into contact with one within 5 to 10 days, the seedling will die. Dodder stems that have attached to a host plant have been known to survive for several days after being detached from the host plant.
As dodder plants grow, they continually reattach to the host. When other suitable hosts are nearby, dodder shoots spread from host plant to host plant, often forming a dense mat of intertwined stems. Shaded areas greatly reduce twining and attachment.
Native Dodder
Native dodder seeds lack obvious dispersal mechanisms and likely spread via people through the movement of soil and equipment or in mud attached to shoes and tires. These seeds also can be transported in infested plant material or be present as a contaminant in crop seed. Water might play a role in seed dispersal, particularly for species near aquatic environments.
Native dodder germinates at or very near the soil surface starting in spring when soil temperatures reach about 60°F. Germination occurs independently of any host plant influence. The germinating seed sends up a slender, twining stem that coils around any object, including host plants.
Seedlings are dependent on carbohydrates stored in the seed (cotyledons) until they attach to a suitable host. When it contacts a host, the stem coils around the host plant and produces little structures called haustoria that penetrate the host’s vascular tissue. The dodder plant begins to extract nutrients and water from the host, and its connection to the soil withers and dries.
Dodder flowers from late spring through fall, depending on the species, but seed set is highest in late summer and fall. Seed production generally begins near the site of initial attachment and proceeds outward. Dodder is a prolific seed producer; each plant is capable of producing several thousand seeds. Generally only about 5% of the seed germinates the year following its production; however, the remainder can remain dormant, yet viable, in the soil for more than 20 years, depending on the species and environmental conditions.
Dodder’s long dormancy is thought to be largely a result of its hard seed coat. To break seed dormancy, scarification—the breaking, scratching, or softening of a seed—usually is required. In nature, this probably occurs through soil microbial activity, weathering, and other natural disturbances such as fire or grazing. In managed settings, scarification likely occurs when fields are cultivated.
Japanese Dodder
Japanese dodder is different from other dodders in that no viable seeds have been observed following flowering in California. Instead, most spread occurs through the dissemination of small pieces of stems distributed by birds and other animals or through pruning, composting, and the improper disposal of infested plant material.
When these stem pieces come in contact with new host plants, they can grow until they cover an entire tree or shrub with their thick, yellow-gold or green stems. Japanese dodder typically grows much larger and faster than other dodders that attack herbaceous plants—up to 6 inches a day.
DAMAGE
Impact varies from moderate to severe reductions of plant growth and, in some cases, complete loss of vigor and death. The severity of an infestation depends on the growth stage of the host plant at the time of initial dodder attachment. With native dodder, the greatest growth reduction occurs when the parasite attaches to seedlings; the infestation usually doesn’t kill established host plants, but when multiple attachments are made to the same host plant, death can occur. Japanese dodder can cover and kill most large shrubs and small trees. The weakened state of infected plants also predisposes them to diseases and insect and nematode invasions.
MANAGEMENT
Japanese Dodder
If you believe you have Japanese dodder in your landscape, do not try to control it yourself. This weed is under an eradication program in California and has spread to more than a dozen California counties including Alameda, Butte, Contra Costa, Fresno, Los Angeles, Merced, Sacramento, Shasta, Solano, Sutter, Tulare, Yolo, and Yuba. Contact your county agricultural commissioner to receive proper identification and help with control. More information is available on the California Department of Food and Agriculture Web site.
Native Dodder
The most successful control involves a systematic approach that combines several methods; you usually can’t eliminate dodder with a single treatment or in a single year. If you see native dodders infesting herbaceous landscape and garden plants, take immediate action to eliminate or reduce the infestation.
Effective management requires control of the current population, prevention of dodder seed production, and suppression of new seedlings in subsequent years. Where extensive infestations exist, remove the infested host plants and replant with nonhosts; in vegetable gardens rotate to nonhost crops for several years. When you plant a host crop again, remove any new dodder plants as soon as possible.
Prevention
The use of dodder-free planting seed has long been a primary way of preventing the spread of dodder infestations. Many countries and states have seed laws that prohibit the presence of dodder seed in planting seed.
Clean and inspect clothing and equipment before moving from infested to “clean” areas. Once you know an area is infested, you must manage it to prevent the further production of dodder seed. Isolate small infestations, and remove them by hand before the plant produces seed. Monitor larger infestations, and mow, prune, burn, or spray herbicides to prevent seed production.
Cultural Control
Planting nonhost plants can be an effective means of managing a dodder infestation. Plants that aren’t hosts of dodder include grasses and many other monocots including lilies. Plants that grow primarily during winter such as crucifers and legumes and transplanted trees and shrubs usually are good alternatives.
Dodder can’t penetrate tree bark, but it can penetrate tree foliage, if it is able to contact it. Be sure to remove weeds in these plantings, so the weeds don’t serve as hosts for dodder and increase the amount of dodder seed in the soil.
Dodder seedlings are difficult to find, but if you see them before they attach to a host, remove them by cultivation or hand pulling. If you see dodder soon after it has attached itself to a host, prune the infected portion of the host plant 1/8 to 1/4 inch below the point of attachment, otherwise the dodder can regenerate from the haustoria left embedded in the host plant. Pruning trees and shrubs generally has been of little benefit unless dodder is confined to one or two branches that you can remove without destroying or disfiguring the entire host plant.
If no host plants are present, you can leave cultivated dodder plants on the soil surface to dry and die. However, if you allow freshly removed dodder to contact a healthy host plant, a new connection sometimes occurs. If the dodder plants have set seed, remove the plants from the area to prevent future infestations. Place plants in a plastic bag, and dispose of them in the trash.
Dodder seed can survive soil solarization—a method for killing weeds using a clear, plastic tarp and the sun’s heat—probably because of its hard seed coat. Although studies haven’t been conducted, composting might kill most dodder seed, because higher temperatures are reached in the composting process than in solarization.
In agricultural settings, cultivate dodder before it attaches to a host plant, since cultivation done after dodder has attached itself to the host is of no benefit. Hand pulling, cutting, or mowing also can reduce dodder infestations. Be sure to break off, cut, or mow the host plant just below the point of dodder attachment (about 1/8 to 1/4 inch) for these methods to be effective. Close mowing is an effective management tool for dodder in alfalfa.
Burning reduces a dodder infestation as long as you destroy the invaded tissue of host plants along with the dodder to prevent regeneration of the dodder from embedded haustoria. Burning kills only some of the dodder seed; the amount of seed destroyed depends on the duration and intensity of the fire.
Biological Control
Several disease organisms are known to infect dodder including Fusarium tricinctum and Alternaria species, which attack swamp dodder (C. gronovii), and A. alternata and Geotrichum candidum, which attack field dodder (C. pentagona). Researchers in China have found that a suspension of Colletotrichum gloeosporioides can selectively control the dodder species C. chinensis and C. australis in soybeans. Difficulty in culturing and applying these organisms has limited their commercialized use.
Resistant Varieties
Breeding programs aimed at developing dodder-resistant varieties are not known to exist; however, some varieties of normally susceptible species have some resistance. Several varieties of processing tomatoes, a plant generally susceptible to dodder, have been observed to be either totally resistant or tolerant to dodder attack.
Chemical Control
Generally chemical control isn’t necessary in the home garden and landscape, since you can control dodder for the most part by cultivating seedlings or through hand removal or pruning. Although pelargonic acid (Scythe) is effective, it also kills any plant tissue it contacts; consequently good coverage and careful spraying are important, so desirable plants aren’t damaged.
Where dodder has been a persistent problem in certain commercial agricultural fields or in landscapes, apply preemergent herbicides (e.g., trifluralin) before dodder seed germinates; where practical, follow up with close mowing, burning, or spot removal of parasitized host plants to control dodder plants that escaped the herbicide application.
Usually postemergent herbicides, which you apply directly to the dodder plant to control it, don’t selectively control dodder without injuring the host plant and aren’t a good choice for controlling established infestations.
REFERENCES
California Department of Food and Agriculture. Noxious Weed Information Project: Japanese Dodder. Accessed March 2010.
Cudney, D. W., S. B. Orloff, and J. S. Reints. 1992. An integrated weed management procedure for the control of dodder (Cuscuta indecora) in alfalfa (Medicago sativa). Weed Technology. 6:603-606.
Cudney, D. W., S. B. Orloff, and D. A. Demason. 1993. Effects of thiazopyr and trifluralin on dodder (Cuscuta indecora) in alfalfa (Medicago sativa). Weed Technology. 7:860-864.
Dawson, J. H., F. M. Ashton, W. V. Welker, J. R. Frank, and G. A. Buchanan. 1984. Dodder and its control. Farmer’s Bull. USDA. No. 2276.
Dawson, J. H., L. J. Musselman, P. Wolswinkel, and I. Dorr. 1994. Biology and control of Cuscuta. Rev. Weed Sci. 6:265-317.
PUBLICATION INFORMATION
Pest Notes: Dodder
UC ANR Publication 7496
Authors: W. T. Lanini, Weed Science/Plant Sciences, UC Davis; D. W. Cudney, Botany/Plant Sciences emeritus, UC Riverside; G. Miyao, UC Cooperative Extension, Yolo Co.; and K. J. Hembree, UC Cooperative Extension, Fresno Co.
Produced by UC Statewide IPM Program, University of California, Davis, CA 95616
PDF: To display a PDF document, you may need to use a PDF reader.
Statewide IPM Program, Agriculture and Natural Resources, University of California
All contents copyright © 2019 The Regents of the University of California. All rights reserved.