success chemistry hair growth reviews
Success Chemistry Hair Growth is a product of a company called Success Chemistry, based in Los Angeles, California. The company has a total of 45 products listed for sale on its website.
Success Chemistry Hair Growth Reviews – Does This Product Really Work?
We, Health Web Magazine, the owner of this e-commerce website, fully intends to comply with the rules of the Federal Trade Commission (FTC) regarding the use of endorsements, testimonials, and general advertising and marketing content. As a visitor to Health Web Magazine you should be aware that we may receive a fee for any products or services sold through this site.
Content
This content may contain all or some of the following – product information, overviews, product specifications, and buying guides. All content is presented as a nominative product overview and registered trademarks, trademarks, and service-marks that appear on Health Web Magazine belong solely to their respective owners. If you see any content that you deem to be factually inaccurate, we ask that you contact us so we can remedy the situation. By doing so, we can continue to provide information that our readers can rely on for truth and accuracy.
Our Top Selections Box – Promotional Sales
Products shown in the section titled ‘Our Top Products’ are those that we promote as the owner and/ or reseller and does not represent all products currently on the market or companies manufacturing such products. In order to comply fully with FTC guidelines, we would like to make it clear that any and all links featured in this section are sales links; whenever a purchase is made via one of these links we will receive compensation. Health Web Magazine is an independently owned website and all opinions expressed on the site are our own or those of our contributors. Regardless of product sponsor relations, all editorial content found on our site is written and presented without bias or prejudice.
Something we believe is that every page on the website should be created for a purpose. Our Quality Page Score is therefore a measurement of how well a page achieves that purpose. A page’s quality score is not an absolute score however, but rather a score relative to other pages on the website that have a similar purpose. It has nothing to do with any product ratings or rankings. It’s our internal auditing tool to measure the quality of the on the page content. There are a number of factors that determine the Page Score of a given page. Landing page quality is a factor in determining Page Score. Landing page quality generally refers to whether or not the overall page contains relevant and original content to the web page visitor. The content quality value of a web page is determined by comparing a page to known quality patterns and each pattern carries a different weighting in how it affects the overall content quality value of a page. We also factor in user generated feedback on this form plus a page quality algorithm. Since web pages content can change, the content quality value of a web page is updated periodically.
Although chemotherapy would help save her life, it would also leave her with no hair. “I promised her that she would have hair,” Paul says. “And when you make a promise to a kid, you keep it.”
WIGS FOR KIDS

About WIGS for kids and their Mission: Helping Children Look Themselves and Live Their Lives!
When Children lose their hair, they don’t just suffer physically. The change in their appearance can drastically undermine their self-image and sabotage their self-esteem. To help heal the pain of these struggles, Certified Cosmetic Therapist Jeffrey Paul founded Wigs for Kids, a nonprofit organization that has been serving children suffering from hair loss since 1981.
Wigs for Kids is a cooperative effort among Certified Cosmetic Therapists throughout North America who share a common goal. “Children shouldn’t have to worry about how they look, especially when they’re in the middle of a health crisis,” says Jeffrey Paul. “We want to give these kids the opportunity to feel good about themselves again.”
The value of all children’s wigs Hair Replacements is $1,800. “These are custom-made Hair Replacements,” says Jeffrey Paul. “Each prosthesis is hand-tied and is made completely from human hair. We make sure they look just like a child’s own hair.”
“They won’t come off on the baseball field or in the playground,” he adds. “Kids can count on them. And because kids look just the way they did before, they feel better about themselves. They look in the mirror and their eyes light up. To see that light in their eyes-that’s priceless.”
Our History:
Looking back over the past 30 years, Wigs for Kids founder Jeffrey Paul cannot believe his incredible journey.
He was a successful hairdresser with a thriving business. He traveled all over the world to work with powerful presidents and gorgeous models. But one day, his 15-year-old niece walked into his salon, crying. She tearfully begged him to stop her hair from falling out. His immediate thoughts were that it was not serious. But when he saw the look in her father’s eyes, he knew it was something more. It turned out that she had just been diagnosed with leukemia. “Uncle Jeff, you know I’ve been trying to get on the gymnastics team all my life,” she cried. “My hair is going to be falling out when it’s time to try out.”
Although chemotherapy would help save her life, it would also leave her with no hair. “I promised her that she would have hair,” Paul says. “And when you make a promise to a kid, you keep it.”
He did some research and learned that designing children’s wigs is complicated because kids are smaller and more active than adults. So, he worked with doctors and prosthetics specialists to devise a hairpiece that would withstand typical kid activities, such as swimming, gymnastics, and sleepovers. They came up with a wig that adhered to the scalp under the most aggressive conditions. And if it got wet, it would look like everybody else’s hair, because every strand of hair was hand-tied.
Paul’s niece was fitted with her wig in time for her gymnastics competition. “My heart was pounding as my wife and I sat in the stands,” he recalls. “And when my niece jumped off the apparatus, she looked up into the stands at us and pointed to her head. Tears ran down my face. I knew that God was taking me to another place in my life. The time was right for me to reach out.”
He got his chance after a local newspaper ran his niece’s story. Wanting to do something good for the community, he asked readers to send him their old wigs, which he would then refurbish and donate to needy patients. “The next day, I received 500 wigs that were beyond repair,” Paul says. “People meant well, but they sent us wigs that had been in their closets since the 1950s. So my wife and I used our own money to start a wig bank.” In no time, word got out that he was helping children and adults who needed wigs.
When a flood destroyed his salon and the insurance company would not cover the damages, he jumped at the chance to open his new business. And he found the perfect space — a medical office that offered the privacy he needed. “When you’re working with somebody who has no hair, you can’t work in an open salon,” he explains. Soon, instead of cutting and styling hair, Paul was custom designing full-cranium prosthetics, or wigs, for children and adults who lost their hair due to medical conditions. To this day, each handcrafted wig is made of about 150,000 strands of natural hair. The individual strands of hair are hand-tied onto the foundation of the wig, which is created from a mold of the person’s head for a snug fit. “I learned on the job and asked some great people to teach me what I didn’t know,” he says. “Quite by accident, I became an innovator.”
Paul didn’t accept hair donations at first. But one day a woman who had cancer came to see him with her daughter who had hair so long she was sitting on it. “Her beautiful, natural blonde hair hadn’t been cut in 18 years,” he explains. “After her mother’s consultation, her daughter said, “Mom, I want to cut my hair for you.” After we all dried our tears, I realized that this child could give her mother nothing more than a part of her body. The mother was so moved that I said I would do it.”
It didn’t take long for the company to grow. “I’d always been an educator and motivational speaker, so I trained people all over the world to do what we were doing: restoring beautiful hair by creating wigs for children, women, and men,” Paul says. He also informed medical professionals and social workers of the hair replacement and children’s wigs options for donating hair to cancer patients.
“There were a lot of kids in need,” he recalls. “The business was getting bigger than we could handle out of our pockets.” So on behalf of Wigs for Kids, he filed for, and received, non-profit status for his charity for children. Now, volunteers sort the donations of hair, answer questions, speak at schools, and hold fundraisers. “We’re a small organization on the inside so we can make a big impact on the outside,” Paul says.
Chemistry Salon is an authorized wigs for kids donation salon! So call today and schedule your appointment 352-479-9999
Safety. No adverse events were reported and no changes were observed during physical examinations.
RESULTS
Efficacy. Subjects were randomized to receive the active medication (N=10) or placebo (N=5). The mean (SD) age of subjects in the active and placebo treatment groups were 49.9 (8.5) years and 47.6 (17.0) years, respectively, and were not significantly different from one another. All subjects described themselves as Caucasian, except one who was Hispanic.
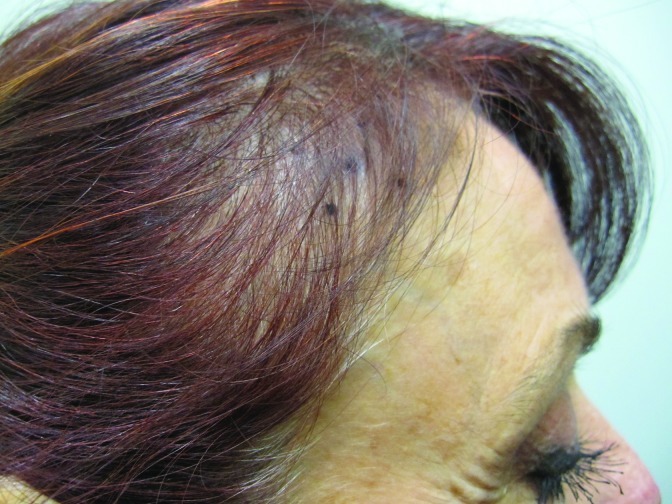
At baseline, the mean number of terminal hairs among placebo-treated subjects was 256.0 (24.1) and remained at 245.0 (22.4) and 242.2 (26.9) after 90 and 180 days, respectively ( Tables 2 ). In contrast, the mean number of terminal hairs in the study medication-treated subjects was 271.0 (24.2) at baseline, increasing to 571 (65.7) and 609.6 (66.6) after 90 and 180 days, respectively (for each, p<0.001 vs. placebo) ( Figure 1 ). Digital images reveal the visible improvements in two subjects treated with the new oral supplement ( Figures 2 – 7 ).
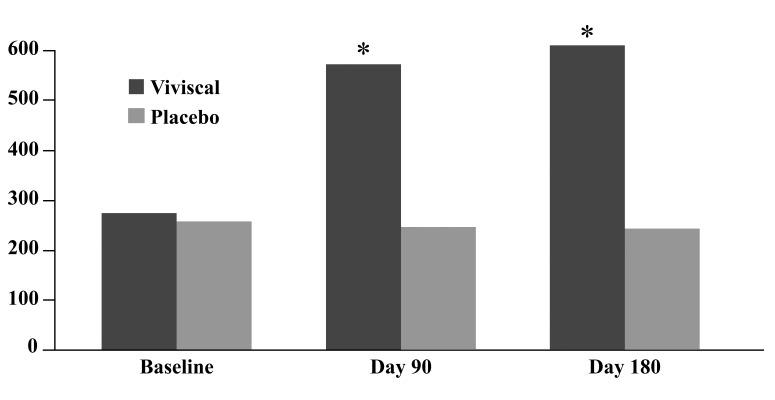
Change in the number of vellus hairs. The use of a new oral supplement was associated with a significant increase in the number of terminal hairs after 90 and 180 days of treatment.
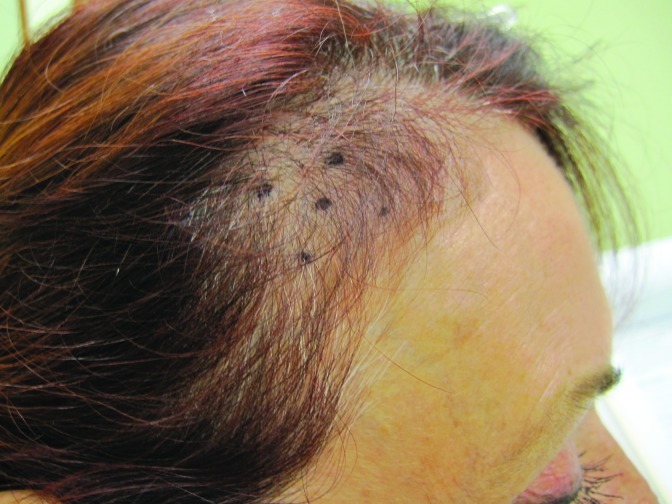
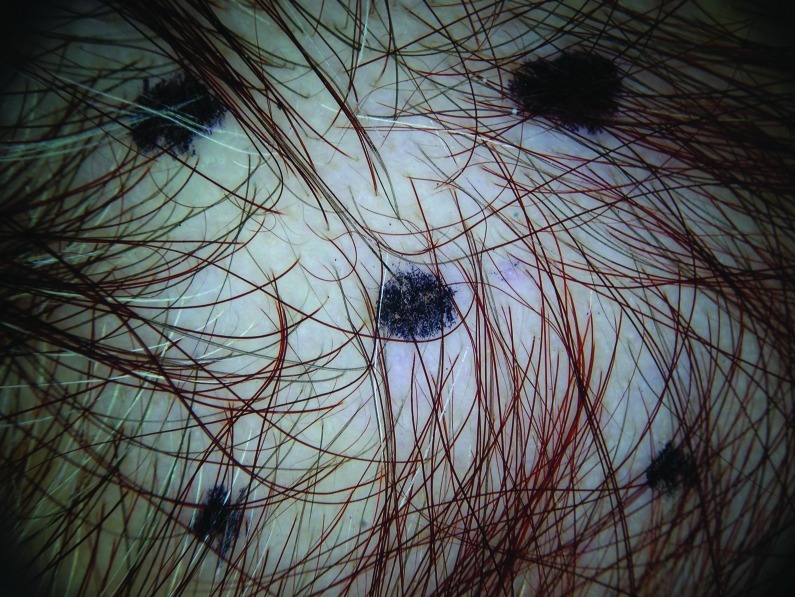
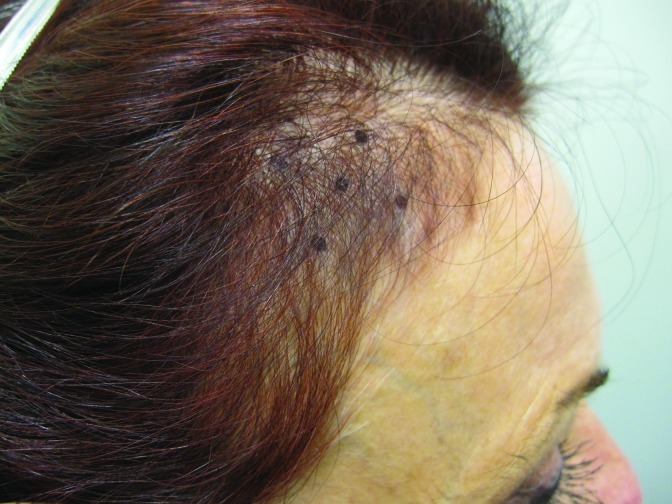
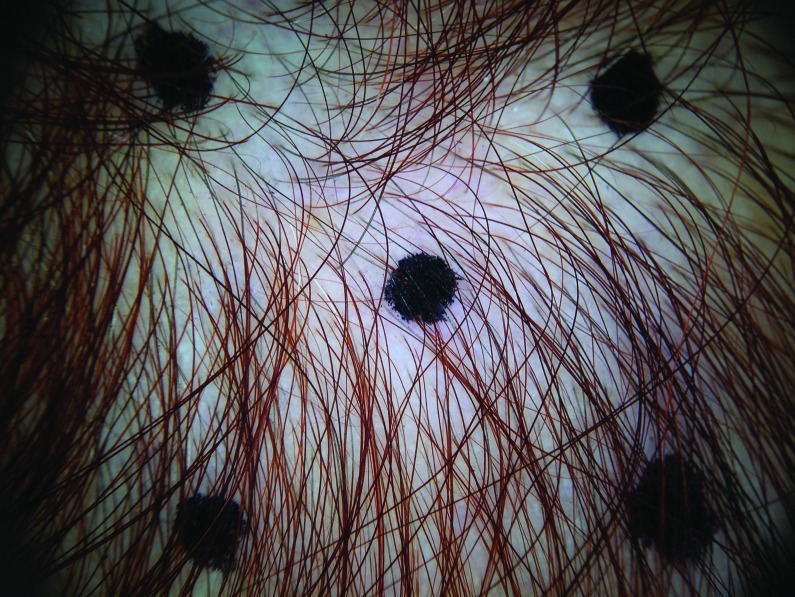
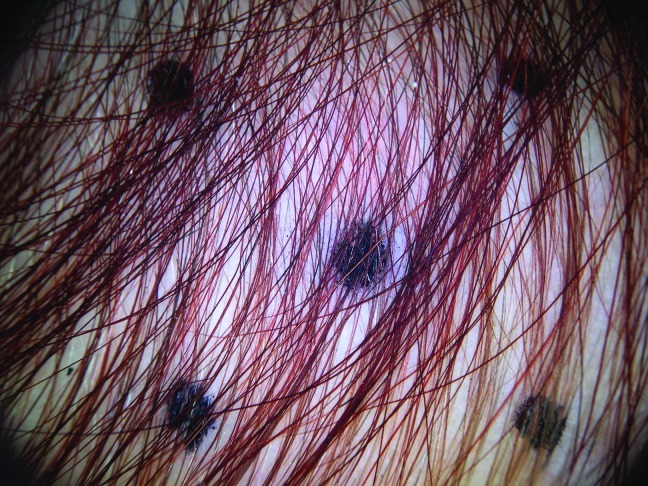
The use of a new oral supplement was associated with a visible increase in hair growth after 90 and 180 days. Figures 2A and 2B = Day 0; Figures 3A and 3B = Day 90; Figures 4A and 4B = Day 180
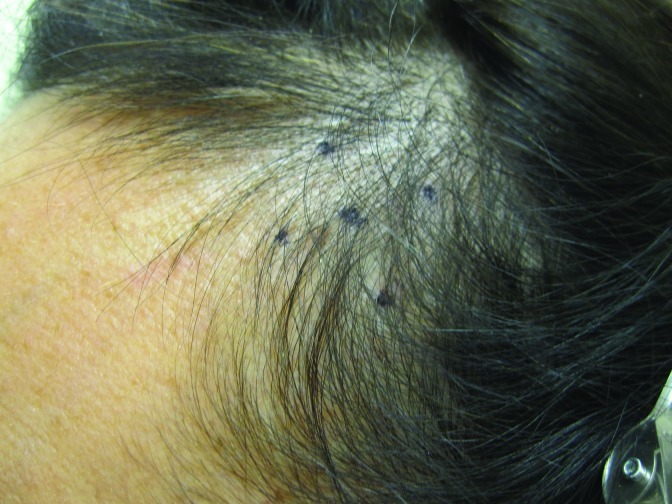
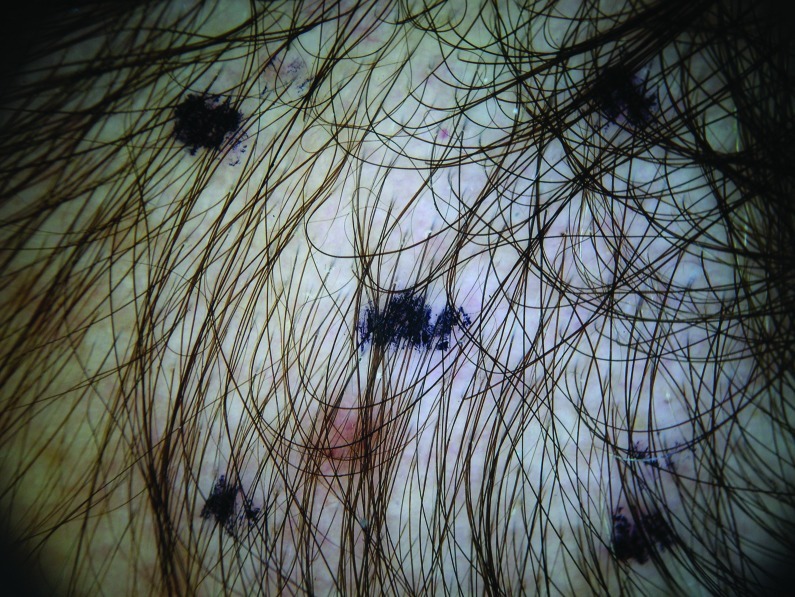
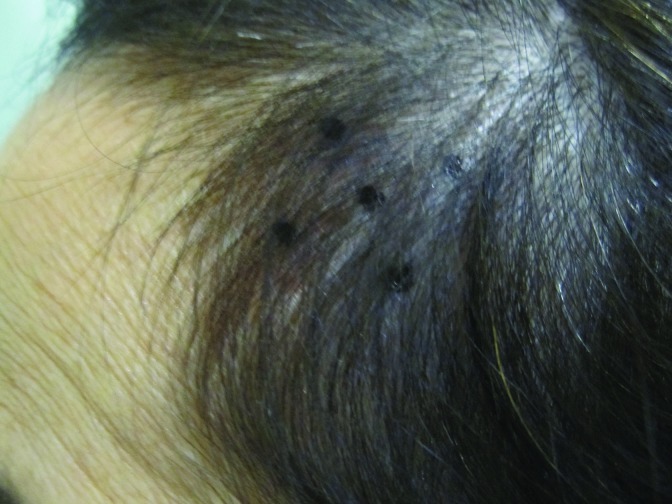

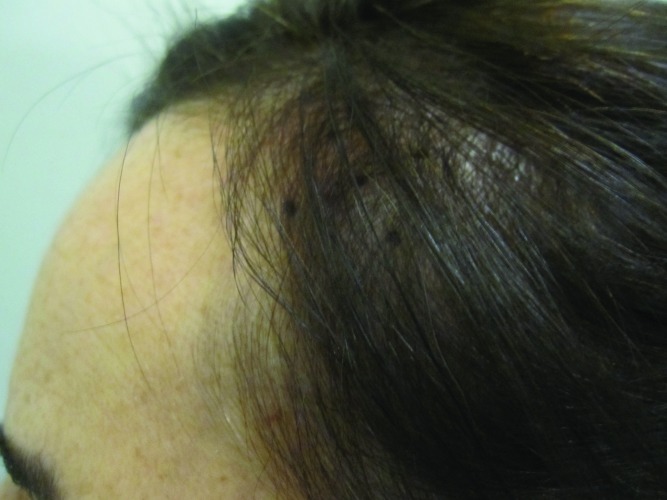

The use of a new oral supplement was associated with a visible increase in hair growth after 90 and 180 days. Figures 5A and 5B = Day 0; Figures 6A and 6B = Day 90; Figures 7A and 7B = Day 180
TABLE 2
Changes in the number terminal and vellus hairs, mean (SD)
| STUDY MEDICATION (N=10) | PLACEBO (N=5) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 90 | Day 180 | Day 0 | Day 90 | Day 180 | |
| Terminal hairs | 271.0 (24.2) | 571.0 (65.7) a | 609.5 (66.6) a | 256.0 (24.1) | 245.0 (22.4) | 242.2 (26.9) |
| Vellus hairs | 46.5 (17.7) | 48.0 (16.2) | 46.5 (14.4) | 57.0 (32.1) | 68.0 (21.4) | 65.8 (16.6) |
The mean number of vellus hairs among placebo-treated subjects was 57.0 (32.1) at baseline and 68.0 (21.4) and 65.8 (16.6) after 90 and 180 days, respectively ( Table 2 ). The mean number of vellus hairs among oral supplement-treated subjects was 46.5 (17.7) at baseline and 48.0 (16.2) and 46.5 (14.4) after 90 and 180 days, respectively.
With respect to subject self-assessments, significantly more study medication-treated subjects perceived improvements in overall hair volume, scalp coverage, and thickness of hair body after 90 days ( Table 3 ). Additional improvements after 180 days included hair shine, skin moisture retention, and skin smoothness.
TABLE 3
Changes in self-assessment questionnaire, mean (SD)
| STUDY MEDICATION (N=10) | PLACEBO (N=5) | |||
|---|---|---|---|---|
| 90 Days | 180 Days | 90 Days | 180 Days | |
| Overall hair volume | 2.8 (0.9) a | 1.8 (0.8) d | 4.2 (0.4) | 3.8 (0.4) |
| Scalp coverage | 2.6 (0.8) b | 1.5 (0.9) d | 4.2 (0.4) | 4.0 (0.0) |
| Thickness of hair body | 2.9 (0.7) c | 2.0 (0.9) d | 4.2 (0.4) | 4.0 (0.0) |
| Hair shine | 2.9 (1.1) | 2.1 (1.3) e | 3.6 (0.9) | 3.6 (0.9) |
| Skin moisture retention | 3.6 (0.8) | 3.0 (0.8) e | 4.0 (0.0) | 4.0 (0.0) |
| Skin smoothness | 3.5 (0.7) | 3.0 (0.8) e | 4.0 (0.0) | 4.0 (0.0) |
Safety. No adverse events were reported and no changes were observed during physical examinations.
This Nutrafol hair review will now go over the pros and cons of the brand.
Nutrafol For Women Review

Nutrafol Women improves hair growth and supports thickness and strength. Its patented Synergen Complex is a proprietary blend of 21 powerful, clinically beneficial ingredients that have been shown to improve hair growth and quantity.
For example, sensoril ashwagandha balances stress hormones to support the hair-growth cycle, while marine collagen provides amino acids as building blocks of hair-strengthening keratin. Get a bottle for $88 or receive regular deliveries for $79/month. Look up Nutrafol Women’s review examples to get the inside scoop from customers.
Nutrafol Women’s Balance Review

Do you want to improve hair growth and get hair with visibly more thickness peri- and post-menopause? Nutrafol Women’s Balance will help you with thinning hair caused by hormones, stress, or metabolism.
This Nutrafol supplement uses medical-grade natural ingredients such as maca, an adaptogen known to support hormone health before, during, and after menopause. Just because your body changes as you get older doesn’t mean your hair has to. Buy a bottle for $88 or schedule deliveries for $79/month.
Many different targeted therapies have been approved for use in cancer treatment. These therapies include hormone therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, immunotherapies, and toxin delivery molecules.
Targeted Cancer Therapies
Targeted cancer therapies are drugs or other substances that block the growth and spread of cancer by interfering with specific molecules (“molecular targets”) that are involved in the growth, progression, and spread of cancer. Targeted cancer therapies are sometimes called “molecularly targeted drugs,” “molecularly targeted therapies,” “precision medicines,” or similar names.
Targeted therapies differ from standard chemotherapy in several ways:
- Targeted therapies act on specific molecular targets that are associated with cancer, whereas most standard chemotherapies act on all rapidly dividing normal and cancerous cells.
- Targeted therapies are deliberately chosen or designed to interact with their target, whereas many standard chemotherapies were identified because they kill cells.
- Targeted therapies are often cytostatic (that is, they block tumor cell proliferation), whereas standard chemotherapy agents are cytotoxic (that is, they kill tumor cells).
Targeted therapies are currently the focus of much anticancer drug development. They are a cornerstone of precision medicine, a form of medicine that uses information about a person’s genes and proteins to prevent, diagnose, and treat disease.
Many targeted cancer therapies have been approved by the Food and Drug Administration (FDA) to treat specific types of cancer. Others are being studied in clinical trials (research studies with people), and many more are in preclinical testing (research studies with animals).
How are targets for targeted cancer therapies identified?
The development of targeted therapies requires the identification of good targets—that is, targets that play a key role in cancer cell growth and survival. (It is for this reason that targeted therapies are sometimes referred to as the product of “rational” drug design.)
One approach to identify potential targets is to compare the amounts of individual proteins in cancer cells with those in normal cells. Proteins that are present in cancer cells but not normal cells or that are more abundant in cancer cells would be potential targets, especially if they are known to be involved in cell growth or survival. An example of such a differentially expressed target is the human epidermal growth factor receptor 2 protein (HER-2). HER-2 is expressed at high levels on the surface of some cancer cells. Several targeted therapies are directed against HER-2, including trastuzumab (Herceptin), which is approved to treat certain breast and stomach cancers that overexpress HER-2.
Another approach to identify potential targets is to determine whether cancer cells produce mutant (altered) proteins that drive cancer progression. For example, the cell growth signaling protein BRAF is present in an altered form (known as BRAF V600E) in many melanomas. Vemurafenib (Zelboraf) targets this mutant form of the BRAF protein and is approved to treat patients with inoperable or metastatic melanoma that contains this altered BRAF protein.
Researchers also look for abnormalities in chromosomes that are present in cancer cells but not in normal cells. Sometimes these chromosome abnormalities result in the creation of a fusion gene (a gene that incorporates parts of two different genes) whose product, called a fusion protein, may drive cancer development. Such fusion proteins are potential targets for targeted cancer therapies. For example, imatinib mesylate (Gleevec) targets the BCR-ABL fusion protein, which is made from pieces of two genes that get joined together in some leukemia cells and promotes the growth of leukemic cells.
How are targeted therapies developed?
Once a candidate target has been identified, the next step is to develop a therapy that affects the target in a way that interferes with its ability to promote cancer cell growth or survival. For example, a targeted therapy could reduce the activity of the target or prevent it from binding to a receptor that it normally activates, among other possible mechanisms.
Most targeted therapies are either small molecules or monoclonal antibodies. Small-molecule compounds are typically developed for targets that are located inside the cell because such agents are able to enter cells relatively easily. Monoclonal antibodies are relatively large and generally cannot enter cells, so they are used only for targets that are outside cells or on the cell surface.
Candidate small molecules are usually identified in what are known as “high-throughput screens,” in which the effects of thousands of test compounds on a specific target protein are examined. Compounds that affect the target (sometimes called “lead compounds”) are then chemically modified to produce numerous closely related versions of the lead compound. These related compounds are then tested to determine which are most effective and have the fewest effects on nontarget molecules.
Monoclonal antibodies are developed by injecting animals (usually mice) with purified target proteins, causing the animals to make many different types of antibodies against the target. These antibodies are then tested to find the ones that bind best to the target without binding to nontarget proteins.
Before monoclonal antibodies are used in humans, they are “humanized” by replacing as much of the mouse antibody molecule as possible with corresponding portions of human antibodies. Humanizing is necessary to prevent the human immune system from recognizing the monoclonal antibody as “foreign” and destroying it before it has a chance to bind to its target protein. Humanization is not an issue for small-molecule compounds because they are not typically recognized by the body as foreign.
What types of targeted therapies are available?
Many different targeted therapies have been approved for use in cancer treatment. These therapies include hormone therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, immunotherapies, and toxin delivery molecules.
- Hormone therapies slow or stop the growth of hormone-sensitive tumors , which require certain hormones to grow. Hormone therapies act by preventing the body from producing the hormones or by interfering with the action of the hormones. Hormone therapies have been approved for both breast cancer and prostate cancer.
- Signal transduction inhibitors block the activities of molecules that participate in signal transduction, the process by which a cell responds to signals from its environment. During this process, once a cell has received a specific signal, the signal is relayed within the cell through a series of biochemical reactions that ultimately produce the appropriate response(s). In some cancers, the malignant cells are stimulated to divide continuously without being prompted to do so by external growth factors. Signal transduction inhibitors interfere with this inappropriate signaling.
- Gene expression modulators modify the function of proteins that play a role in controlling gene expression.
- Apoptosis inducers cause cancer cells to undergo a process of controlled cell death called apoptosis. Apoptosis is one method the body uses to get rid of unneeded or abnormal cells, but cancer cells have strategies to avoid apoptosis. Apoptosis inducers can get around these strategies to cause the death of cancer cells.
- Angiogenesis inhibitors block the growth of new blood vessels to tumors (a process called tumor angiogenesis). A blood supply is necessary for tumors to grow beyond a certain size because blood provides the oxygen and nutrients that tumors need for continued growth. Treatments that interfere with angiogenesis may block tumor growth. Some targeted therapies that inhibit angiogenesis interfere with the action of vascular endothelial growth factor (VEGF), a substance that stimulates new blood vessel formation. Other angiogenesis inhibitors target other molecules that stimulate new blood vessel growth.
- Immunotherapies trigger the immune system to destroy cancer cells. Some immunotherapies are monoclonal antibodies that recognize specific molecules on the surface of cancer cells. Binding of the monoclonal antibody to the target molecule results in the immune destruction of cells that express that target molecule. Other monoclonal antibodies bind to certain immune cells to help these cells better kill cancer cells.
- Monoclonal antibodies that deliver toxic molecules can cause the death of cancer cells specifically. Once the antibody has bound to its target cell, the toxic molecule that is linked to the antibody—such as a radioactive substance or a poisonous chemical—is taken up by the cell, ultimately killing that cell. The toxin will not affect cells that lack the target for the antibody—i.e., the vast majority of cells in the body.
Cancer vaccines and gene therapy are sometimes considered targeted therapies because they interfere with the growth of specific cancer cells. Information about cancer vaccines can be found in NCI’s Cancer Treatment Vaccines page.
How is it determined whether a patient is a candidate for targeted therapy?
For some types of cancer, most patients with that cancer will have an appropriate target for a particular targeted therapy and, thus, will be candidates to be treated with that therapy. CML is an example: most patients have the BCR-ABL fusion gene. For other cancer types, however, a patient’s tumor tissue must be tested to determine whether or not an appropriate target is present. The use of a targeted therapy may be restricted to patients whose tumor has a specific gene mutation that codes for the target; patients who do not have the mutation would not be candidates because the therapy would have nothing to target.
Sometimes, a patient is a candidate for a targeted therapy only if he or she meets specific criteria (for example, their cancer did not respond to other therapies, has spread, or is inoperable). These criteria are set by the FDA when it approves a specific targeted therapy.
What are the limitations of targeted cancer therapies?
Targeted therapies do have some limitations. One is that cancer cells can become resistant to them. Resistance can occur in two ways: the target itself changes through mutation so that the targeted therapy no longer interacts well with it, and/or the tumor finds a new pathway to achieve tumor growth that does not depend on the target.
For this reason, targeted therapies may work best in combination. For example, a recent study found that using two therapies that target different parts of the cell signaling pathway that is altered in melanoma by the BRAF V600E mutation slowed the development of resistance and disease progression to a greater extent than using just one targeted therapy (1).
Another approach is to use a targeted therapy in combination with one or more traditional chemotherapy drugs. For example, the targeted therapy trastuzumab (Herceptin) has been used in combination with docetaxel, a traditional chemotherapy drug, to treat women with metastatic breast cancer that overexpresses the protein HER2/neu.
Another limitation of targeted therapy at present is that drugs for some identified targets are difficult to develop because of the target’s structure and/or the way its function is regulated in the cell. One example is Ras, a signaling protein that is mutated in as many as one-quarter of all cancers (and in the majority of certain cancer types, such as pancreatic cancer). To date, it has not been possible to develop inhibitors of Ras signaling with existing drug development technologies. However, promising new approaches are offering hope that this limitation can soon be overcome.
What are the side effects of targeted cancer therapies?
Scientists had expected that targeted cancer therapies would be less toxic than traditional chemotherapy drugs because cancer cells are more dependent on the targets than are normal cells. However, targeted cancer therapies can have substantial side effects.
The most common side effects seen with targeted therapies are diarrhea and liver problems, such as hepatitis and elevated liver enzymes. Other side effects seen with targeted therapies include:
- Skin problems (acneiform rash, dry skin, nail changes, hair depigmentation)
- Problems with blood clotting and wound healing
- High blood pressure
- Gastrointestinal perforation (a rare side effect of some targeted therapies)
Certain side effects of some targeted therapies have been linked to better patient outcomes. For example, patients who develop acneiform rash (skin eruptions that resemble acne) while being treated with the signal transduction inhibitors erlotinib (Tarceva) or gefitinib (Iressa), both of which target the epidermal growth factor receptor, have tended to respond better to these drugs than patients who do not develop the rash (2). Similarly, patients who develop high blood pressure while being treated with the angiogenesis inhibitor bevacizumab generally have had better outcomes (3).
The few targeted therapies that are approved for use in children can have different side effects in children than in adults, including immunosuppression and impaired sperm production (4).
What targeted therapies have been approved for specific types of cancer?
The FDA has approved targeted therapies for the treatment of some patients with the following types of cancer (some targeted therapies have been approved to treat more than one type of cancer):