bronson reactiv 8 reviews
Other Ingredients: Modified cellulose, microcrystalline cellulose, magnesium stearate, silicon dioxide.
ReActiv-8™ Hair Formula – 60 Vegetarian Capsules

FREE Standard Shipping on US Orders of $9.99 and more!
15% Off $40 with code INSTANT15
25% Off $75 with codeINSTANT25
- Promotes healthy hair
- DHT blocking
Why ReActiv-8
The most common type of hair loss is Androgenetic Alopecia (AGA). Pattern hair loss is believed to result from the conversion of testosterone to DHT (dihydrotestosterone) by the 5-alpha-Oreductase (5AR) enzyme. DHT attach to the hair follicles, causing a negative change in the growth pattern of hair. The hair shrinks in diameter and the hair growth cycle becomes shorter. The result is smaller, thinner, virtually invisible hairs and shrinking of hair coverage on the scalp.
Our formula can help! We have formulated a product containing LinumLife®, which has clinical studies to prove results are “real” and that this 5-alpha reductase inhibitor has a very high rate of efficacy for a DHT blocker and inhibitor. LimuLife® is a nutritional ingredient product from a flaxseed that is rich in compounds called lignans. Lignans can help balance hormone levels in the body. Helping balance hormone levels in the body, the barrier to block DHT becomes superior and new hair growth begins.
About Bronson
Since 1960, Bronson has been providing families with the highest quality products and finest customer service available. This 60-year heritage speaks for itself. But with so many online vitamin stores, why should you purchase supplements from Bronson?
In short, our company is time proven, offering the purest, high quality vitamins backed by science. Trust Bronson to provide the very best natural health products for you and your family.
| Product Name | ReActiv-8™ Hair Formula – 60 Vegetarian Capsules |
| SKU | 163A |
| UPC | 716563163013 |
| Price | $27.99 |
| Shipping Price – Standard | 0.00 |
| Weight | 0.120000 |
| Brand | Bronson Vitamins |
| Size | 60 Vegetarian Capsules |
| Form | Vegetarian Capsule |
| Lifestage | Adult |
| Serving Size | 2 Vegetarian Capsules |
| Suggested Frequency | Daily |
| Servings Per Container | 30 |
| Days supply | 30 |
| Unit Count | 60 |
| Unit Count Type | Vegetarian Capsules |
| Item Condition | New Condition |
| Product Pill Size | Capsule C2 |
| California Proposition 65 | No |
| Directions | As a dietary supplement for adults, 1 capsule twice daily, preferably with meals, or as directed by a health professional. |
Directions: As a dietary supplement for adults, 1 capsule twice daily, preferably with meals, or as directed by a health professional.
| Supplement Facts Serving Size: 2 Vegi-Caps Servings Per Container: 30 | ||
| Amount Per Serving | % Daily Value | |
| LinumLife® EXTRA Flax Seed Extract (standardized for 20% lignans) | 250 mg | † |
| Gingko Biloba Leaf Extract (standardized for 24% flavone glycosides and 6% terpene lacones) | 240 mg | † |
| Bilberry Fruit Extract (standardized for 25% anthocyanosides) | 240 mg | † |
| Green Tea Extract (standardized for 98% polyphenols, 70% total catechins and 45% EGCG) | 200 mg | † |
| Grape Seed Extract (standardized for > 80% phenolic content | 50 mg | † |
| † Daily Value Not Established. | ||
Other Ingredients: Modified cellulose, microcrystalline cellulose, magnesium stearate, silicon dioxide.
WARNING: Please consult a healthcare professional before taking this product if you are pregnant or nursing.
Do not use if seal under cap is broken or missing.
Store at room temperature.
Keep out of reach of children.
To ensure our website performs well for all users, the SEC monitors the frequency of requests for SEC.gov content to ensure automated searches do not impact the ability of others to access SEC.gov content. We reserve the right to block IP addresses that submit excessive requests. Current guidelines limit users to a total of no more than 10 requests per second, regardless of the number of machines used to submit requests.
More Information
Internet Security Policy
By using this site, you are agreeing to security monitoring and auditing. For security purposes, and to ensure that the public service remains available to users, this government computer system employs programs to monitor network traffic to identify unauthorized attempts to upload or change information or to otherwise cause damage, including attempts to deny service to users.
Unauthorized attempts to upload information and/or change information on any portion of this site are strictly prohibited and are subject to prosecution under the Computer Fraud and Abuse Act of 1986 and the National Information Infrastructure Protection Act of 1996 (see Title 18 U.S.C. §§ 1001 and 1030).
To ensure our website performs well for all users, the SEC monitors the frequency of requests for SEC.gov content to ensure automated searches do not impact the ability of others to access SEC.gov content. We reserve the right to block IP addresses that submit excessive requests. Current guidelines limit users to a total of no more than 10 requests per second, regardless of the number of machines used to submit requests.
If a user or application submits more than 10 requests per second, further requests from the IP address(es) may be limited for a brief period. Once the rate of requests has dropped below the threshold for 10 minutes, the user may resume accessing content on SEC.gov. This SEC practice is designed to limit excessive automated searches on SEC.gov and is not intended or expected to impact individuals browsing the SEC.gov website.
Note that this policy may change as the SEC manages SEC.gov to ensure that the website performs efficiently and remains available to all users.
Note: We do not offer technical support for developing or debugging scripted downloading processes.
The NICoE 4-week intensive outpatient program (IOP) is comprised of an interdisciplinary care model. The NICoE receives referrals from providers throughout the Military Health System for patients who have not responded to conventional treatments and who present with persistent symptoms from service-related injuries. Service members engage in a full-time schedule of patient-provider encounters across approximately 25 different disciplines. Patients are able to access up to 25 different services, and providers coordinate treatment within the interdisciplinary team. Patients undergo extensive neuroimaging and diagnostic evaluations to address presenting symptomatology from TBI and PTSD. This includes chronic pain and disruptions pertaining to personal, occupational, and familial functioning. The interdisciplinary model, which encompasses creative arts therapies (e.g., music therapy, art therapy, dance/movement therapy, and therapeutic writing) as a standard of care, is fundamental to the success of the NICoE.
Music Therapy Program Models
The music therapy programs at the NICoE and ISFB are supported by the NEA Creative Forces Military Healing Arts Network. At these sites, music therapy programming—alongside other creative arts therapies—is standard of care within the interdisciplinary patient-centered model, optimizing comprehensive treatment and recovery processes. Music therapy is utilized as a targeted and stand-alone treatment as well as in co-treatment with other disciplines to support patient goals in the realms of speech and language, cognition, motor coordination, social integration and engagement, psychological health, and spousal/family support. All music therapy sessions are facilitated by board-certified music therapists. We first present a brief description of the clinical setting to help contextualize music therapy program models. This is followed by a presentation of typical goal areas and associated music therapy interventions used for treatment. We then present the specific models of music therapy employed at NICoE and ISFB.
Description of Clinical Settings.
The National Intrepid Center of Excellence at Walter Reed National Military Medical Center.
The NICoE is a clinical research facility that offers high-quality, innovative treatment of TBI and psychological health conditions for service members and families.
Intensive outpatient program.
The NICoE 4-week intensive outpatient program (IOP) is comprised of an interdisciplinary care model. The NICoE receives referrals from providers throughout the Military Health System for patients who have not responded to conventional treatments and who present with persistent symptoms from service-related injuries. Service members engage in a full-time schedule of patient-provider encounters across approximately 25 different disciplines. Patients are able to access up to 25 different services, and providers coordinate treatment within the interdisciplinary team. Patients undergo extensive neuroimaging and diagnostic evaluations to address presenting symptomatology from TBI and PTSD. This includes chronic pain and disruptions pertaining to personal, occupational, and familial functioning. The interdisciplinary model, which encompasses creative arts therapies (e.g., music therapy, art therapy, dance/movement therapy, and therapeutic writing) as a standard of care, is fundamental to the success of the NICoE.
Longitudinal care.
The music therapy program at the NICoE may provide continuing care to non-IOP patients who have varying levels of TBI. Patients are referred to music therapy by medical professionals such as doctors, nurses, social workers, case managers, medical staff, occupational therapists, and speech language pathologists, or are self-referred. Music therapy uses a standardized assessment process consisting of provider referrals, patient eligibility, and presence of non-musical goals that can be addressed through music. For each referral, the music therapist consults with other providers, such as the service member’s nurse case manager, to determine eligibility, necessity, and projected effectiveness of music therapy services. The music therapist reviews additional information (i.e., medical records) to glean insight regarding injury and schedules a music therapy assessment to determine an appropriate treatment plan. This then leads to either patient intake for continued services, referral to other resources such as recreational music programs on base or in the community, or recommendation of a hybrid program. Long-term TBI outpatients receive individual music therapy sessions at the frequency of one session per week for 60, 90, or 120 minutes, as determined by the music therapist and treatment team. Due to individualization of long-term music therapy treatment, the IOP program will be the focus of the NICoE music therapy program presented in this article.
Intrepid Spirit Center at Fort Belvoir Community Hospital.
ISFB opened in September 2013 as the first Intrepid Spirit Center, serving the local military population in a long-term, outpatient treatment setting. ISFB is a department under the Fort Belvoir Community Hospital, which serves all military branches and is the second largest military treatment facility in the National Capital Region. It serves active-duty military and veterans with mild to moderate TBI and their families. Each patient is individually evaluated by multidisciplinary professionals, and receives his or her own holistic treatment plan. The team uses a wide variety of interventions to address the patients’ complex needs.
University model.
ISFB uses an innovative treatment model similar to a university education curriculum, known as the Intrepid Spirit University. In this model, patients and providers customize a curriculum with treatment and educational courses at the core. This model focuses on five pillars: sleep, nutrition, physical movement, pain management, and adaptive resiliency ( Fort Belvoir Community Hospital, n.d.). The five pillars serve as a focused approach for goal setting, treatment planning and implementation, and evaluation of patient progress. An individualized curriculum is aimed at mitigating stress, promoting beneficial neuroplasticity, and empowering the patient to engage in his or her recovery. Patients collect course credits and work toward a commencement ceremony marking the completion of their treatment. This model optimizes treatment, provides a clear path and timeline for both clinician and patient, and sets expectations for patient to reach clinical goals.
Music Therapy Goal Areas with Associated Music Therapy Interventions
Music therapy interventions implemented at NICoE, ISFB, and other military installations where Creative Forces music therapists work are evidence based and research informed. Music therapy interventions are geared toward skill building, stress management, and improving functional outcomes. Music therapy is integrated into varying program models—IOP and longitudinal care—to meet the diverse needs and goal areas of the patient population. Elements of military service can impact length of treatment; therefore, strategic selection of interventions requires consideration by the therapist of how to support best possible outcomes for each patient. Neurologic Music Therapy interventions are incorporated into treatment to improve function in areas of cognition, motor responses, and speech-language comprehension and execution ( Thaut & Hoemberg, 2014), alongside interventions targeted at treatment of behavioral health issues and psychoeducation for patients and their families. Music therapy can simultaneously address and uniquely treat functionality and emotional health by attending to interactions of symptomatology that accompanies co-morbid diagnosis. Figure 1 outlines common goal areas for the treatment of patients with co-morbid TBI and PTSD diagnosis.
MG is a life-threatening adverse event of acute onset and rapid progression after ICI initiation. Early use of IVIG or PLEX, regardless of initial symptoms severity, may lead to better outcomes than steroids alone. Our data suggest the need to reassess the current recommendations for management of ICI-related MG until prospective longitudinal studies are conducted to establish the ideal management approach for these patients.
Results
A total of 5898 patients received ICI at MD Anderson. Among these, 14 (0.24%) were diagnosed with MG. Of 10,442 unique articles from the literature, 46 publications describing 53 patients met inclusion criteria (Additional file 2: Figure S1), including two patients who had been identified from MD Anderson [21]. Therefore, a total of 65 patients were included in our final analysis; 58 fulfilled the criteria for a definite diagnosis of MG and the remaining patients had probable MG.
Patient characteristics
Patient demographic and baseline characteristics are shown in Additional file 3: Table S1. The median age was 73 years (range: 34 to 86 years); 42 (65%) were males, and the most common type of cancer was melanoma (48%). Most patients received anti-PD-1 therapy (82%). A preexisting diagnosis of MG was reported in 13 patients (20%). Clinical information for each patient along with the quality appraisal of the cases retrieved from the literature are provided in Additional file 4: Table S2 and Additional file 5: Table S3 respectively.
ICI-related MG
Of the 65 identified patients, 63 (97%) developed MG symptoms following ICI initiation (52 developed new onset MG and 11 had a flare of their preexisting MG). Overall, 41 (63%) developed moderate to severe muscle weakness (MGFA class III to V) after ICI (Table 1). The most frequent symptoms were ptosis (75%), dyspnea (62%), limb weakness (55%), dysphagia (48%), and diplopia (42%). Concurrent diagnosis of myositis was noted in 24 patients (37%), and myocarditis in five (8%); two had the triad of MG/myositis/myocarditis (Fig. 1). Median time from ICI initiation until the first MG symptom was 4 weeks (range: 6 days – 16 weeks) (Fig. 2). Respiratory failure requiring mechanical ventilation occurred in 29 patients (45%), including 12 who initially presented with severe respiratory compromise, and 17 who progressed to myasthenic crisis after initiation of MG treatment. Patients with MG/myositis/myocarditis seemed to develop respiratory failure more than those with MG only (54% vs. 42%). The median time from first MG symptom till respiratory failure was 7 days (range: 24 h – 60 days) (Fig. 3).
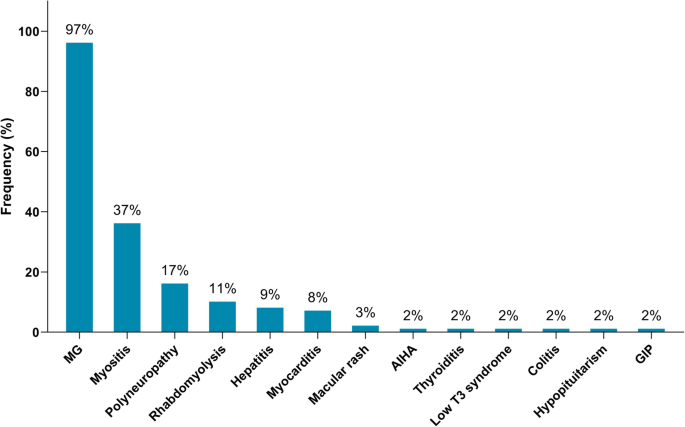
Immune-related adverse events diagnosed in patients following initiation of ICI therapy (n = 65). MG = myasthenia gravis; AIHA = autoimmune hemolytic anemia; GIP = granulomatous inflammation of the pleura
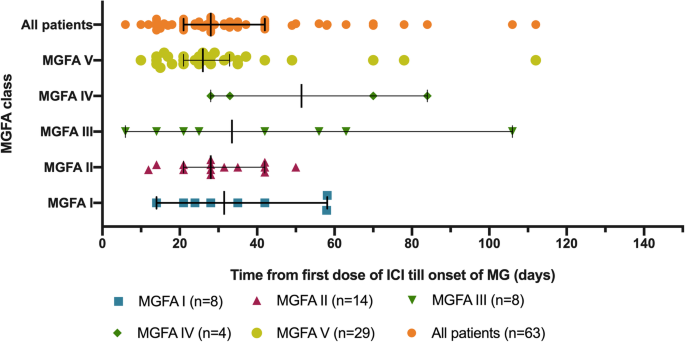
Time from first infusion of immune checkpoint inhibitor to onset of first MG symptom. ICI = immune checkpoint inhibitor; MG = myasthenia gravis; MGFA = Myasthenia Gravis Foundation of America
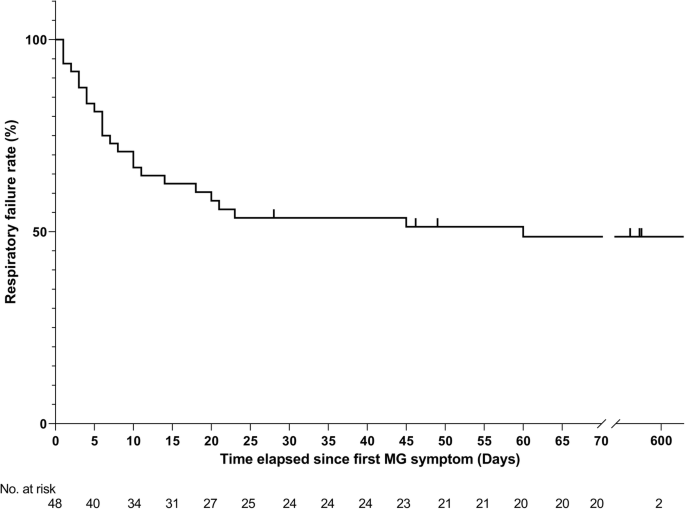
Kaplan-Meier curve for respiratory failure. Of the 63 patients who developed symptoms of myasthenia gravis following initiation of checkpoint inhibitors, the time elapsed since first MG symptom and/or the date of the last follow-up was not available for 15 patients. MG = myasthenia gravis
Diagnostic features
Elevation of anti-AChR antibodies was reported in 37/56 tested patients (66%) (median: 1.64 nmol/L, range: 0.05–98 nmol/L) (Table 1). Of note, three patients were found to have positive AChR antibody in retrospective blood samples that were drawn before ICI initiation, but their antibody titers increased at least 2-fold after ICI [22,23,24]. Anti-striated antibodies were detected in 12/18 tested patients (67%), and 41/49 tested patients (84%) had elevated creatine phosphokinase (CPK) (median 2638 IU/L, range: 418 to 19,794 IU/L). Those with elevated CPK seemed to develop respiratory failure more than those with normal levels (56% vs. 38%). Details on other diagnostic tools are summarized in Additional file 6: Table S4.
Electrodiagnostic studies were performed in 37 patients and detected features of MG in 15 (41%), and both MG and myopathy in six (16%). Computed tomography was negative for thymoma, and magnetic resonance imaging excluded brain metastasis or acute intracranial events. Transthoracic echocardiography showed left ventricular dysfunction in four patients (27%) who had an overlapping diagnosis of myositis/myocarditis, while electrocardiography showed diffuse ST elevation, premature ventricular contractions, right bundle branch block, or ventricular tachycardia in five others (34%).
Skeletal muscle biopsy was performed in seven patients and showed inflammatory infiltrates in five (71%). Three others had myocardial biopsy, which revealed inflammatory infiltrates in all. The inflammatory infiltrates in both skeletal and myocardial biopsies consisted of CD8+ and CD4+ T lymphocytes as well as B lymphocytes and macrophages.
Management and outcomes
Of the 63 patients who developed ICI-related MG, 96% required hospitalization. Overall, corticosteroids (3–1000 mg/day) were used in 59 patients (94%) (Table 1). Acetylcholinesterase inhibitors were used in 32 (51%), intravenous immunoglobulin (IVIG) in 30 (48%), plasmapheresis (PLEX) in 28 (44%), and other immunosuppressants in 10 (16%). Additionally, invasive ventilation was used in 12 patients (19%) and non-invasive positive pressure ventilation in 14 (22%); three others refused intubation and opted for palliative care. Only 10 patients were successfully weaned from mechanical ventilation including three who still required oxygen therapy. One patient with myocarditis also required temporary implantation of a pacemaker [25], and another one required intervention with an intra-aortic balloon pump [26]. Discontinuation or withholding of ICI was recommended in 61 patients (97%), the remaining two continued ICI after resolution of symptoms with steroids [27].
Overall, MG symptoms completely resolved in 12 patients (19%), improved in 34 (55%), and worsened in 16 (26%) (Table 1). Information on both the sequence of treatments for MG and outcome at last follow-up were available for 59 patients. Of 38 patients who received steroids only as first line therapy, 24 (63%) had improvement of symptoms. In the remaining 14 patients who progressed to respiratory failure, IVIG or PLEX was added as a second line therapy for 12 patients but with no improvement. Of note, four of these 14 patients had initially presented with ocular symptoms but eventually progressed to myasthenic crisis after initiation of steroid (ranging from 30 mg to 1000 mg per day). In contrast, of 19 patients who received IVIG or PLEX (regardless of steroid) as first line, 18 (95%) had improvement of symptoms (p = 0.011) (Fig. 4). Of note, the upfront use of IVIG or PLEX in those patients may have been triggered by early development of severe respiratory/bulbar symptoms in 17 patients, and may have been based on the prescriber’s preference in two others who presented with only mild symptoms. Additionally, one patient with ocular symptoms was treated by holding ICI, and another one with mild weakness was treated by acetylcholinesterase inhibitor leading to improvement. Data on maintenance therapy for MG after discharge were available for 31 patients. Of those, 26 (84%) were on steroid taper protocols, 10 (32%) were receiving acetylcholinesterase inhibitors, five (16%) IVIG, one mycophenolic acid, and another one rituximab. Death was reported in 24 patients (37%), primarily because of MG complications in 15 patients (23%) after a median of 6 weeks (range: 3–26.5 weeks) of the initial MG symptoms. Of the 15 patients who died because of MG complications, two had MG alone, and 13 had elevated CPK levels including nine who were diagnosed with MG overlapping with myositis/myocarditis. Overall, patients who were tested for CPK and/or troponin seemed to have a higher MG deterioration rate than those who were not tested (29% vs. 13%), and a higher mortality rate primarily because of MG complications (29% vs. 6%) (Additional file 7: Table S5).
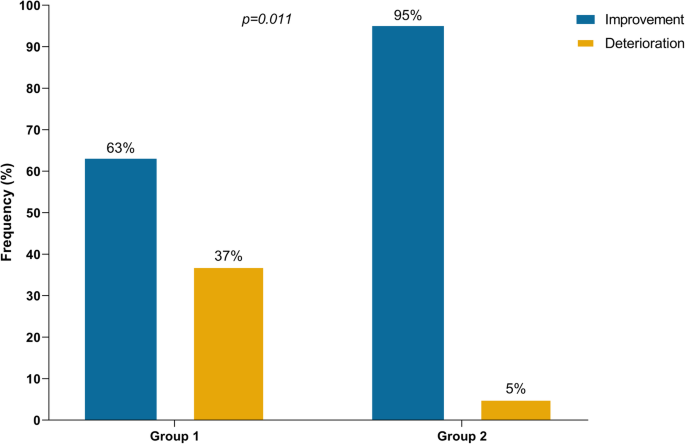
Outcomes of immune checkpoint inhibitor-related myasthenia gravis according to first-line treatment. Group 1: Patients who received steroids without concurrent intravenous immunoglobulin or plasmapheresis in first-line treatment (n=38). Group 2: Patients who received intravenous immunoglobulin or plasmapheresis regardless of steroids in first-line treatment (n=19)
Information on the tumor response to ICI was available for 20 patients with melanoma. Ten (50%) achieved partial or complete response, five (25%) had stable disease, while five others (25%) had tumor progression.
Notably, the clinical presentation, diagnostic findings, management, and clinical outcomes of ICI-related MG did not differ when we excluded the patients who were identified from the literature (Table 1) nor the patients diagnosed with probable MG from our cohort (Additional file 8: Table S6).
Rechallenge or continuation of ICI treatment
Re-administration of ICI was reported for six patients after resolution of MG symptoms. In three the initial MG symptoms were limited to ocular symptoms or mild weakness, while the other three had more severe weakness. After symptom resolution, all patients were maintained on prednisone, pyridostigmine and/or IVIG at ICI re-administration. Time from the first MG symptom until ICI re-administration ranged from 7 days to 17.75 months. Five patients were treated with the same initial agent (anti-PD-1) and one switched from ipilimumab to pembrolizumab. Upon ICI re-administration, none of the patients had recurrence of symptoms. Two of these patients eventually had partial or complete tumor response, one had stable disease, and three had progressive disease.
Patients with preexisting MG
Thirteen patients had pre-existing MG; eight (67%) were treated with immunosuppressants (steroids, mycophenolate mofetil, azathioprine), IVIG, and/or acetylcholinesterase inhibitor before ICI initiation. The time interval between diagnosis of MG and ICI initiation was 5.3 years (1–20 years). At ICI initiation, modification of the baseline treatment (reducing immunosuppression dose and/or acetylcholinesterase inhibitors or adding IVIG) was recommended for four patients, and only one had active preexisting MG symptoms. Of the 13 patients, 11 (85%) had disease flare after ICI initiation. One patient (8%) developed ocular symptoms (MGFA classes I) and ten others (76%) developed more severe weakness (MGFA III, IV and V) (Table 2). MG flare was fatal in two patients including one who was receiving maintenance therapy at ICI initiation. There were no significant differences in median times from ICI initiation to onset of MG symptoms, MGFA class, clinical manifestations, diagnostic findings, management, nor clinical outcomes between patients with preexisting MG and those with new onset disease that manifested clinically only after ICI initiation (Table 2).
Only two patients with preexisting MG did not show any signs of disease exacerbation after ICI initiation. Both had no active MG symptoms at ICI initiation and were maintained on prednisone 10 mg or pyridostigmine 120 mg. Of 5 melanoma patients, 3 (80%) achieved partial response.
Patients with ICI-related MG compared to idiopathic MG (iMG)
Patient demographics, MGFA classification, time to class IV/V, rate of MG/myositis/myocarditis overlap, and type of autoantibodies observed in patients with ICI-related MG compared to iMG are shown in Table 3. MGFA class IV/V MG occurred in more than half of our patients (51%) which is much higher than what has been recently reported in patients with iMG (2–10%) [26, 28]. The median time from first MG symptom to class IV/V was 7 days (range: 24 h to 60 days) in our cohort, while in those with iMG, progression to class IV/V typically occurs within 2–3 years [29, 30]. The overlap with myositis/myocarditis was also much higher compared to patients with iMG (42% vs. 0.9%) [31]. Additionally, positive anti-striated muscle antibodies were more frequently observed in our patients compared to non-thymoma patients with iMG [32].